Electric charge is one of the fundamental concepts in electronics. Learning the electric charge definition is crucial to understanding other basic concepts of electronics.
Electric charge is the fundamental or intrinsic property of subatomic particles of an atom. These subatomic particles are positively charged protons and negatively charged electrons. It is important to learn the electric charge to better understand electricity and how electronic devices work because the movement of these charges produces electricity.
In this article, we’ll discuss in detail about electric charge and laws related to it.
Electric charge
As we know, an atom is composed of subatomic particles called electrons, protons, and neutrons.
Our eyes have the property to see, and ears to listen to sounds. Similarly, subatomic particles have a property called electric charge.
Protons and electrons have electrical charges positive and negative while neutron has no charge. Because of this positive and negative charges atoms attract or oppose each other.
You can observe electric charges in your daily life by rubbing different materials together. For example, when you rub the balloon on your hair.
Due to rubbing the hair loses electrons and becomes positively charged (i.e., deficiency of electrons). On the other hand, a balloon takes these electrons and becomes negatively charged (i.e., it has an excess of electrons).
Because of this, charge difference is created, remember the opposite attracts, so your hair is attracted to the balloon due to the difference in charge.
Now we can define electric charge.
Electric charge is a property of matter that shows attraction or repulsion when it comes in contact with another charged matter.
We can also say that electric charge is produced due to the transfer of electrons.
Electric charge is also defined as the property of matter that causes it to experience a force when placed in an electric and magnetic field.
Types
The electric charge has two main types which are positive and negative.
1. Positive charge
Protons, which are present in the nucleus of an atom, have a positive charge. Protons have a positive electric charge, typically denoted by the symbol “+”.
2. Negative charge
The negative charge is associated with an electron, which is the particle of the atom that revolves around the nucleus. Electrons carry a negative electric charge, conventionally represented as “-”.
The positive and negative charge plays a vital role in understanding the behavior of electric charges. The opposite charges, such as positive and negative attract each other.
On the other hand, the same charges as negative negative, and positive positive repel each other.
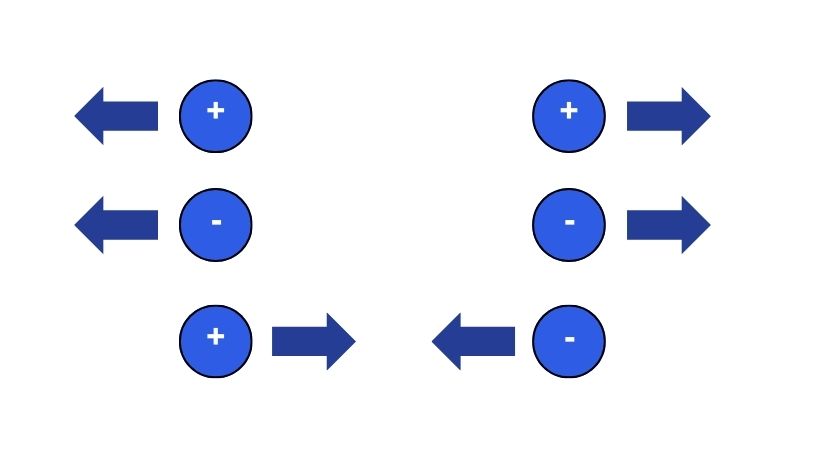
When an object has more electrons than protons then it has a negative charge. The opposite occurs when there are more protons than electrons, it is positively charged.
When two objects have an equal number of positive and negative charges they are in the neutral state because they cancel out each other.
Measurement
let’s see how can you measure the electric charge and what is its unit.
Electric charge is a scalar quantity means it can be completely described by its magnitude and symbolically electric charge is represented by “q” and “Q”. The SI unit of electric charge is coulomb (C).
Mathematically the electric charge can be measured by:
Q = Ne
Where “Q” is electric charges, “N” refers to the number of electrons and protons, and “e” is constant and represents the charge on an electron or a proton in the system. The value of e is 1.602*10^(-19).
I will give you an example of how will you calculate the electric charge. Suppose we have a charged object with 1 electron then the total charge on the body is:
Q = 1*(-1.602*10^(-19)) C
Q = -1.602*10^(-19) C
The negative sign shows that the charge on the object is due to electrons. If it is due to proton it will have a positive sign.
The electric charge can also be measured in terms of electric current and time. Electric current is the flow of electric charges.
If a uniform current flows in the circuit, the following equation represents the charges that pass through the circuit.
Q=I.t
The “I” in the equation represents electric current and “t” shows the time.
Properties
To study the properties of electric charges, a very small electric charge also known as a point charge is considered.
Let’s discuss some properties of electric charges.
1. Conservation of charges
The conservation of charges means that a charge can not be created nor destroyed. It always transfers from one body to another, but it cannot be created or destroyed.
In other words, if there are two bodies or objects in a system then one body has positive charges so the other body will have the same number of negative charges.
For example, if the balloon is rubbed with hair, it becomes negatively charged and hair becomes positively charged by an equal amount.
The charges always adhere to the restriction that the net quantity of charge must remain the same.
2. Additivity of electric charges
The electric charges are scalar quantities if it is considered point charges. The additivity property of charges says if there are “n” number of charges, the total charges present will be the algebraic sum of the charges.
Q=Q1+Q2+………….+Qn
3. Quantization of charge
Quantization of charge means the charges are transferred in packet form, or they come in discrete, indivisible units called elementary charges.
The charge carried by an electron, which is around -1.6*10^-19 coulombs, is the smallest unit of electric charge. This quantization of charge means electric charge cannot be broken up into smaller parts.
Coulomb’s law
We have studied electric charges repel each other if the charges are the same and attract each other if they differ. However, we don’t know how much force they apply to each other during attraction and repulsion.
Coulomb’s law provides the method to calculate the force between charges.
Coulomb’s law was provided by French physicist Charles Augustin de Coulomb, he carried out a lot of experiments to find the force between electric charges.
We have two charges “q1” and “q2” and the distance between them is denoted by “r”.
Coulomb’s law states,
“The magnitude of the force between two point charges is directly proportional to the product of the magnitude of the charges and inversely proportional to the square of the distance between them.”
Mathematically presentation:
According to the definition, the force of magnitude is directly related to the magnitude of charges.
F ∝ q1q2
Inversely related to the square of the distance between them.
F ∝ 1/r^2
Now combine both equations.
F ∝ q1q2/r^2
Then,
F =K( q1q2/r^2)
Where “K” is the constant proportionality and its value depends upon the nature of the medium between charges. The value of “K” is generally expressed in terms of the permittivity of free space.
K=1/4πε_o
The symbol ε_o is the permittivity of a vacuum and its value is 8.85410^-12 C^2 N^-1 m^-2.
We can quantify the strength of the electric force between charges and learn important information about how they interact by using Coulomb’s Law.
1. Coulomb’s law in a material medium
The above derivation of Coulomb’s law is valid when the medium between charges is air or vacuum. It is experimentally noted that force decreases when an insulator is placed between electric charges.
The magnitude of the force between two electric charges is affected by the property of the medium called the permittivity of the medium. So Coulomb’s law for the material medium is given by:
F_med =(1/4πε)*(q1q2/r^2)
This ε is called the permittivity of the medium. The permittivity of the medium as compared to the permittivity of the vacuum is called relative permittivity “ε_r”. The ε_r is equal to:
ε_r =ε/ε_o
ε =ε_r*ε_o
So the equation of force for material medium becomes:
F_med =(1/4πε_rε_o)*(q1q2/r^2)
F_med =1/ε_r{(1/4πε_o)*(q1q2/r^2)}
In the above equation, the right side shows the force between two electric charges in air or vacuum.
Then,
F_med=F_vac/ε_r
This equation shows that if the medium between electric charges changes the force between them will be affected by the relative permittivity of the medium.
Methods of charging
Charging is supplying an object with an electric charge or removing an electric charge from an object.
An object having no charge can be charged by various methods.
1. Charging by friction
Charging by friction means rubbing an object with another object. When one object is rubbed over another object, the transfer of electrons takes place due to the friction between two objects.
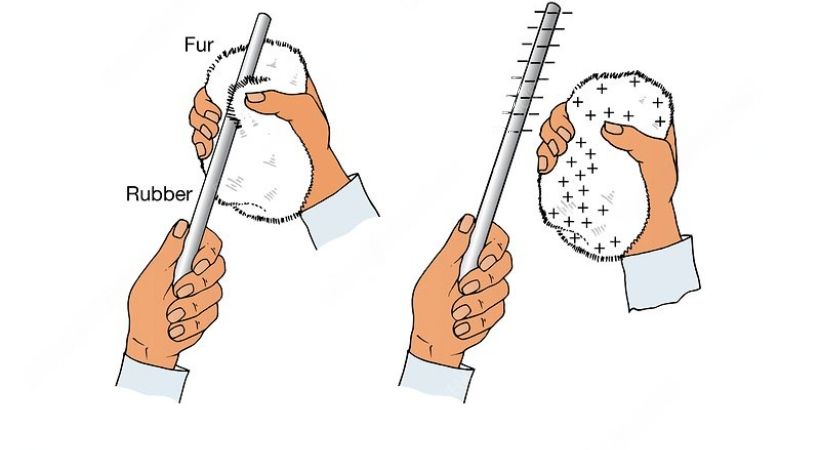
For example, when we rub a rod with fur, the fur loses electrons and becomes positively charged and the rod becomes negatively charged by gaining electrons.
2. Charging by induction
Charging by induction means by inducing charges on an uncharged object by bringing a charged object near to it, but not touching it.
Consider a rod that is negatively charged and a metal sphere which are neutral.
When the rod is brought near the sphere, the positive charges in the sphere travel to the end of the sphere closest to the plastic rod due to attraction to the plastic rod.
Similar to positive charges, negative charges are attracted to the other end of the sphere from the plastic rod and migrate there.
As a result, the charges within the sphere rearrange themselves so that all positive charges are now closer to the plastic rod and all negative charges are now farther away.
3. Charging by conduction
Charging by conduction is charging an uncharged object by bringing it in contact with another charged object.
When a charged object is brought in contact with an uncharged object, the electrons are transferred from the charged object to the uncharged object because a charged object has an unequal number of negative and positive charges.
For example, consider two metal rods A and B. Rod B is negatively charged when brought in contact with uncharged rod A.
The transfer of negative charge from rod B to rod A takes place so that rod A becomes negatively charged by gaining electrons and rod B becomes positively charged by losing electrons.
Conclusion
As a beginner of learning electronics, electric charge is more than a concept. The invisible thread that binds the fields of science, technology, and daily life together is called electric charge.
Electric charge is the intrinsic property of matter when it comes in contact with charged matter showing attraction and repulsion. There are two types of electric charges, positive and negative.
The negative charge is related to electrons and the positive charge is related to protons. Electric charges possess some properties like conservation and additivity.
Coulomb’s law emerged as a cornerstone in our understanding of electric charge.
This law explains how charged objects interact, describing the attractive forces between particles with opposite charges and the repulsive forces between particles with similar charges.
An uncharged object can also be charged by various methods such as charging by friction, conduction, and induction.
This is all about electric charge and I hope as a beginner you find this article beneficial.
Other useful posts:
